Holding the new Dexcom G7 CGM

AppleInsider may earn an affiliate commission on purchases made through links on our site.
After months of international availability, the Dexcom G7 constant glucose monitor received its FDA clearance to distribute in the US. Ahead of the wide rollout, we got to go hands-on with a demo model to see how it looks and fits.
Initially, Dexcom had hoped to launch the G7 much earlier here stateside. But a massive rewrite to the mobile app and sensor algorithms prompted increased scrutiny from the FDA before it could begin sales.
It launched first abroad before the FDA clearance came in early December. The device is still unavailable to order in the US but will start hitting pharmacies in early 2023.
Now that the final certification is in, we got a demo model that shows off the final form factor, insertion process, and how it compares to the G6.
What's new
The Dexcom G7 is known as a CGM, or constant glucose monitor. It sends readings every five minutes from a tiny sensor under your skin to your smartphone or a receiver via Bluetooth.
With the G7, Dexcom has continued to refine the product with several improvements over the G6.
- Smaller design
- Faster warmup period
- New mobile app with Clarity reporting built in
- 12-hour grace period for migrating to a new sensor
- New wear locations
- Less plastic waste
- New insertion device
- Better, more customizable alerts

The biggest of the changes is the new design, which is 60 percent smaller than its predecessor. It's an all-in-one design that combines the previously-separate transmitter and sensor.
This requires a new insertion tool that looks more akin to the Freestyle Libre with a button on the side. The whole unit is disposable, no longer requiring the transmitter to be reused.
It connects to the redesigned G7 app, which is yet to release in the US. The new app combines the Dexcom Clarity app with the CGM app so you can view your CGM data and reports in one location.
The new app has more granular alerts too. These new alerts can be personalized to your needs and can be more discrete.
The Dexcom G7 only requires a 30-minute warm-up, compared to two hours with the G6, and still requires no manual fingerstick calibration.
Dexcom allows a 12-hour grace period when the sensor expires, which is excellent for parents with children. They no longer have to decide between sending them to school with a sensor about to expire and ending the wear session early.
Finally, the G7 is approved to be worn in two new body locations. The G6 was only supposed to be worn on the abdomen. The G7 can be worn on the arm for those older than two, and on the upper buttocks for kids ages two through 17.

Post-launch, Dexcom has told AppleInsider that it plans to allow the G7 to connect directly to Apple Watch without requiring the iPhone as an intermediary.
This will allow users to leave their iPhone behind, possibly for a workout, and still receive glucose readings on their Apple Watch.
Practical use of the Dexcom G7
We've been using the Dexcom G6 CGM for some time, making it an easy comparison to the new G7. The size difference is deceivingly minuscule when holding them side-by-side.
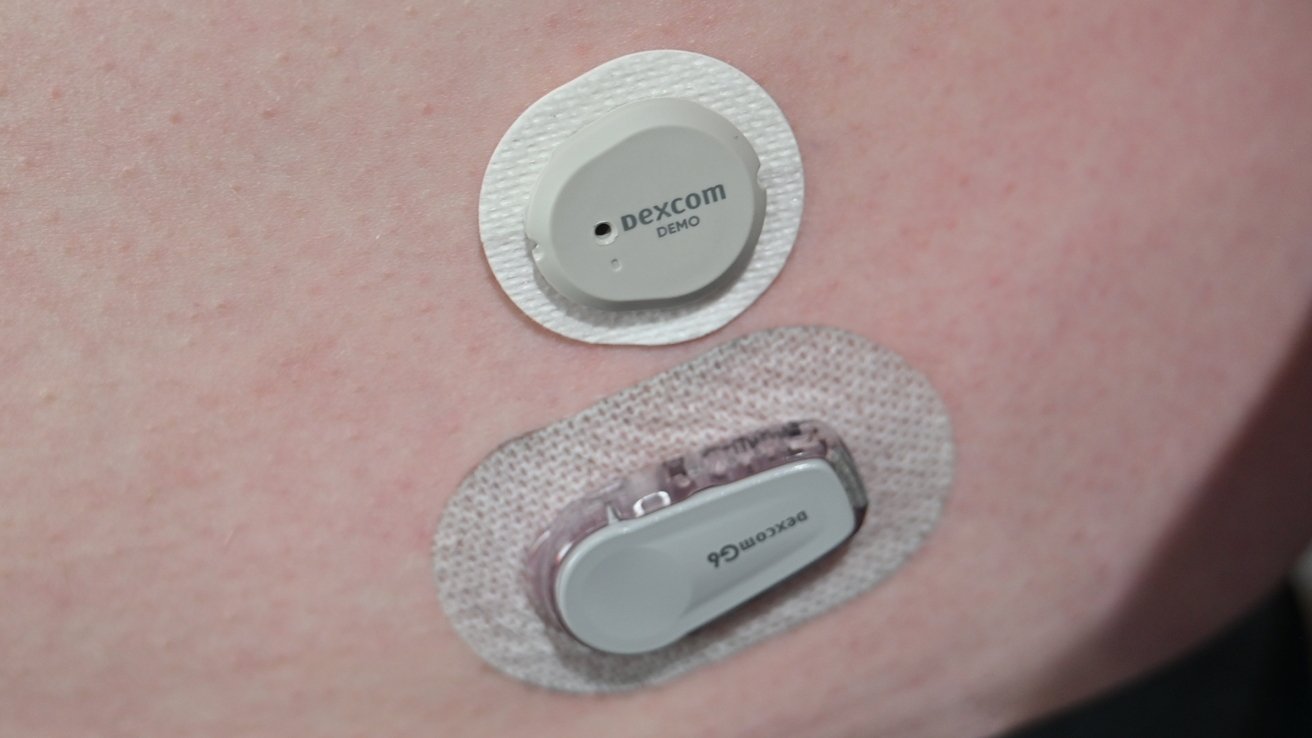
As the Dexcom G6 must go in a clip, its size is noticeably larger when worn. The G7 also has beveled edges, allowing clothing to pass more easily on top of it without getting caught.
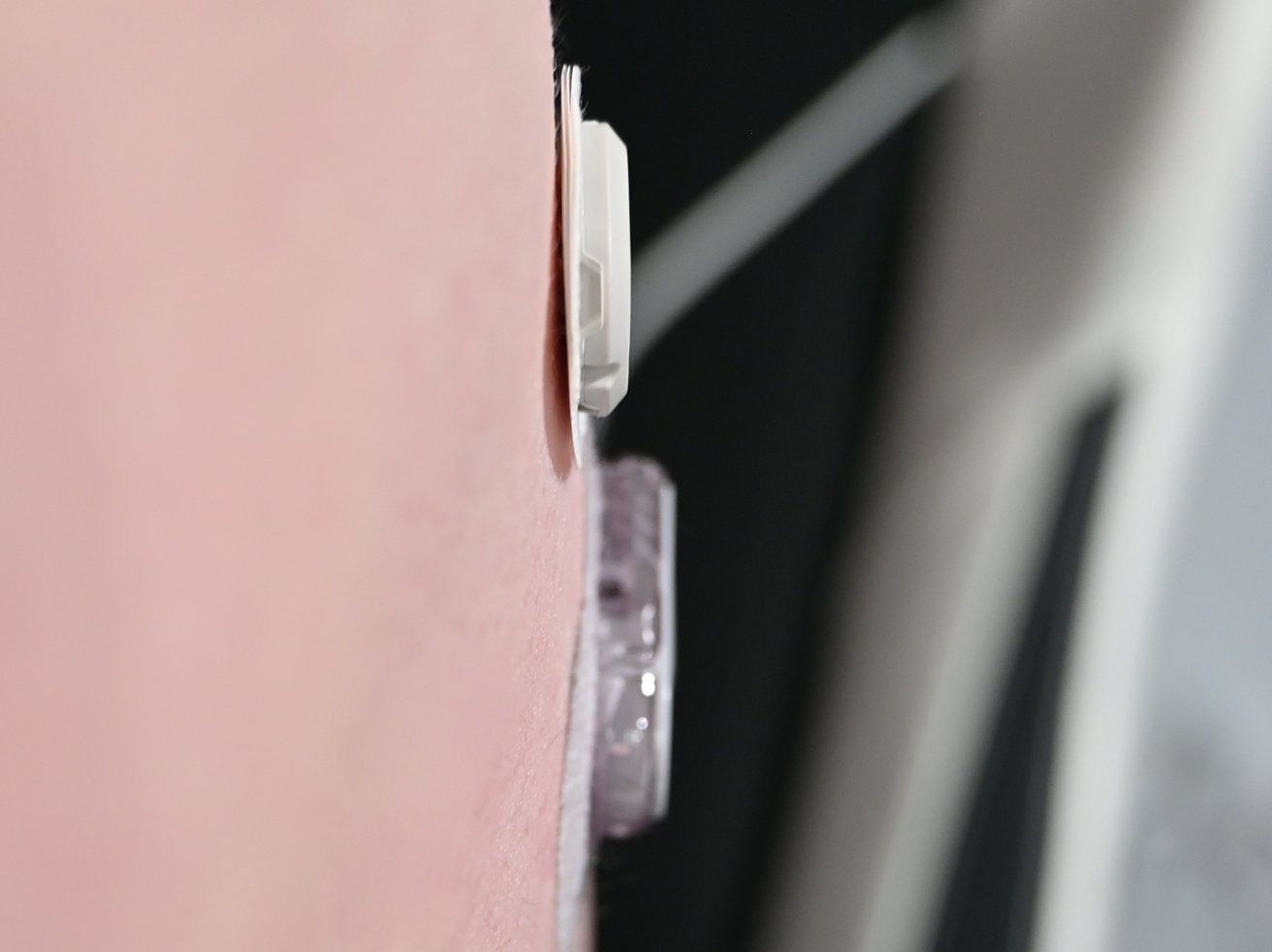
In our sample photo above, you can see how much more slim the G7 is when just placed on the skin. It will sit more flush with the adhesive applied.
The G7 has a soft-touch finish that is almost rubber-like but isn't as tacky as rubber or silicone. The G6 has a hard, slippery exterior.

We like the new insertion tool that is even easier to use than the G6 model. The sensor is magnetically held in place and inserted with a button.
A cap is screwed onto the bottom, making for quick disposal.
Coming in 2023
Our demo model is non-functioning, meaning we still need to test the accuracy or connectivity features.
The app and sensor will begin shipping in early 2023 and requires a prescription to order. Dexcom says it is continuing to work with insulin pump providers, such as Tandem, to support the new sensors as fast as possible.